Approach
The approach to managing iron deficiency and iron deficiency anemia involves:
Identification of iron deficiency or iron deficiency anemia
Investigating and managing the underlying etiology
Iron repletion

Iron Repletion
Iron repletion can be achieved using either oral or intravenous iron formulations.
When choosing the route of iron supplementation, the advantages and disadvantages of each should be considered, along with the patient’s health-related quality of life, and the patient should be engaged in shared decision making.
Oral Iron vs Intravenous Iron

Oral Iron
Oral iron supplementation is considered as first line in the treatment of mild iron deficiency in stable outpatients in whom oral iron is well tolerated, as it is inexpensive and easy to access. To maximise the efficacy, oral iron should be taken between meals and medications that reduce gastric acidity, such as antacids, should be avoided when the iron supplement is taken. Vitamin C can enhance the absorption.

Adverse Reactions Associated with Oral Iron
Adverse effects include:
Nausea
Vomiting
Constipation
Diarrhea
Dark stool
Metallic taste

Strategies to reduce the adverse effects of oral iron
There are strategies to reduce the incidence of these adverse effects, such as:
Choosing formulations with less elemental iron content
Alternate day dosing
Ingestion of the iron supplement with meals

Responses to Oral Iron Supplementation
If there is no ongoing blood loss, the expected response is an increase in hemoglobin at an average of 10g/L per 7-10 days, and generally should be continued for at least 3 months, and then response should be reassessed with CBC, reticulocyte count and ferritin levels. Reticulocytosis starts in 4-5 days, peaking after 7-10 days, and hemoglobin begins to improve by the second week (at an average of 10 g/L per week). Anemia will not resolve with ongoing blood loss or malabsorption of oral iron.
If patients do not respond to oral iron, adherence should be assessed, the diagnosis of iron deficiency should be reconfirmed, and parenteral iron should be considered.

Oral Iron Dosing & Formultations
It is important to note that with the exception of heme iron polypeptide formulations, oral iron supplementation dosing should not exceed once daily dosing, as this will increase the risk of adverse effects, which interferes with adherence and may even result in decreased absorption due to increasing hepcidin levels.
Common Oral Iron Formulations Available in Canada and the United States

Intravenous (IV) Iron
IV iron was previously only used when oral iron was not effective or rapid replacement was required, but now it's preferred over oral iron in many populations because of other advantages:
Rapid onset efficacy
Improved adherence
Acceptable safety profile
Indications to Use IV Iron as First Line

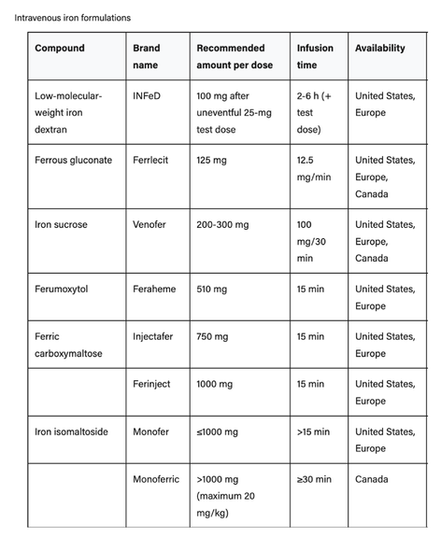
IV Iron Formulations & Dosage
Common IV Iron Formulations
The choice of IV iron formulation and dosage depends on availability, cost, and the severity of iron deficiency and anemia.
Intravenous iron is not universally covered by provincial drug programs in Canada nor by private insurance programs.
The Ganzoni Formula, which calculates the patient's total body iron deficit based on weight and current hemoglobin levels, can guide dosing choice of IV iron formulation. Ganzoni Formula (weight in Kg x (15 – current Hb g/dL] x 2.4 + 500), helps determine which IV iron formulation or dose is most suitable. A common approaches for different stages of iron deficiency would be using a smaller dose formulation for iron deficiency without anemia or mild IDA (Hb >110 g/L), and larger dose formulations for more severe iron deficiency (e.g. Hb ≤ 110 g/L).
Prior to each IV iron infusion and after 3 months, a CBC, reticulocyte count and ferritin levels (with iron profile if there is inflammation) should be tested to assess if iron stores have been replenished and if ongoing supplementation is necessary.
The aim is to prevent symptoms of iron deficiency and continue treatment until ferritin levels >100 microgram/L and TSAT >30%.
The aim is to prevent symptoms of iron deficiency and maintain normal iron status, indicated by ferritin levels >50 microgram/L and TSAT >20%.

Adverse Reactions Associated with IV Iron
Adverse reactions include:
Fatigue, headache, myalgia, and low-grade fever. This typically occurs within 1-2 days of the infusion, and self-resolves.
Brown discoloration of the urine. This occurs immediately after the infusion.
Skin staining. This occurs if there is extravasation of the IV iron.
Hypersensitivity reactions. These have an incidence of less than 0.1% (4) and include Fishbane reaction, infusion reaction, and isolated symptoms.
Anaphylactic reactions. These have an incidence less than 1 in 200,000. The risk is minimal compared to the risk of serious adverse events with RBC transfusions, which are estimated to be 1 in 40,000.

Risk Of Infection From IV Iron
Therapeutic iron doses may increase the risk of infection due to iron promoting microbial growth of sideophilic microbes. Therefore, it is reasonable to avoid using parenteral iron in patients with active sepsis, especially if sepsis is due to siderophilic bacteria. (5) In a recent meta-analysis, there was an increase in the risk of infection with intravenous iron (relative risk 1.16, 95% CI 1.03-1.29) compared with oral iron or no iron supplementation. Previous meta-analyses have found either no or a similar small increase in risk of infection with IV iron.

Red Blood Cell Transfusion
The use of red blood cell transfusion should be restricted to cases with debilitating symptoms or cardiovascular compromise.
Choosing Wisely campaigns and the American Society of Hematology advise against transfusing RBCs for iron deficiency in the absence of hemodynamic instability, and promote the use of alternative therapeutic options.